Ultimate Guide to Etching Specialty Alloys for Microstructural Evaluation
Technical Guide
A Guide to Etching Specialty Alloys for Microstructural Evaluation
Growing use of specialty alloys with high resistance to corrosion has challenged many laboratories to refine the way they etch alloy samples for microstructural evaluation. The big problem – the alloys resist the etchants just as they do the corrosive conditions encountered in service.
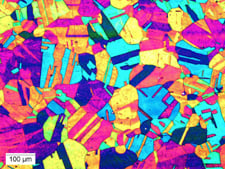 |
| Microstructure of a stainless 330 sample in annealed condition @ 100x, using a tint etch consisting of a solution of 40 ml hydrochloric acid (MCL) + distilled water (H2O) + one gram potassium meta bisulfite (K2S2O5) + 4 grams ammonium biflouride (NH4F–HF) at room temperature. |
Such high-performance alloys have been used increasingly for critical applications in bio-medical, aerospace and defense industries, among others. Due to the usually demanding material requirements, laboratory evaluation of the finished microstructure is often desired, if not required.
The difficulty in etching these materials has focused attention also on employing the most effective procedures for etching a wide variety of iron-, nickel- and cobalt-based alloy systems. Ensuing discussion, therefore, will offer guidelines for processing stainless steels, high temperature alloys, tool and alloy steels, and magnetic and expansion alloys.
Background
Light microscopy to evaluate the microstructure of metals is employed extensively by quality control and failure analysis laboratories. Selection of the proper etchant depends largely upon alloy composition, heat treatment and processing. The etchants used in metallographic examination are solutions of acid and chemicals and are applied selectively to attack a highly polished surface, thus permitting microstructural examination.
There are three basic methods of etching alloy samples – immersion, swabbing and electrolytic. In the first method, the sample is immersed in the etching solution until the desired structure is developed. Samples may be immersed in stain etchants to highlight specific microstructural features.
In the second, the sample is swabbed with cotton that has been immersed in the etchant. In electrolytic etching, a D.C. source or a rectifier serves as the power supply, and the specimen is the anode in the electrolytic cell. Power requirements can be adjusted as needed, depending on sample size, anode-to-cathode spacing, electrolyte, etc.
The following rules should be observed to obtain a true and representative microstructure:
- If a mounted specimen is used, an adherent mount is very important. Separation between the specimen and the mounting compound can result in “bleeding” of the residual etchant or water, and subsequent staining.
- A good metallographic polish is a must. The sample must be free of scratches, disturbed metal and any kind of embedded contaminants.
- The specimen must be thoroughly cleaned before etching. Oil or any polishing compounds must be removed.
- After etching, the sample should be rinsed in HOT water, followed by an alcohol rinse and dried under HOT air. (Note: an alcohol rinse can dull/wash out a stain etch.) Samples with cracks must be thoroughly dried to prevent bleeding.
- If additional etching time is required, the specimen should be re-rubbed a few seconds on a final polishing wheel. If this is not done, “flashing” can occur in certain alloys, necessitating a complete re-polish. Flashing will not occur and re-rubbing is not required if the alloy being etched forms no passive oxide layer, or if etching is performed electrolytically (rerubbing is usually mandatory only when using Ralph’s, Glyceregia, Acetic Glyceregia and HCL + H2O2 etchants). More complete specimen preparation instructions are readily available in literature.
Etchants & Alloys
Table 1 lists 28 etchants commonly used in the laboratory, along with their compositions and guidelines for use. Table 2 lists various specialty alloys, along with the suggested etchants for each and application notes. These tables can be used to quickly select the appropriate etching procedure for the alloys most often used today. Additional etchants are reported in ASTM specification E-407-99 “Standard Practice for Microetching Metals and Alloys” and ASM Metals Handbook (Volume 9, Metallography and Microstructures).
The etchants for each alloy are presented in order of preference and successful experience. In general, the most benign etchant is shown first, followed by those that grow progressively stronger. If a weaker etchant is tried first and it does not yield satisfactory results, the investigator only has to buff the surface slightly to obtain a good polish for examination with a stronger etchant.
If the first etchant attempted is too strong, far more preparation is required to restore the surface to a workable condition. If one is not familiar with the etchants recommended, it is usually a good idea to start with the weakest solution.
Before etching, the examiner should first consider what s/he is looking for. If the intent is to evaluate non-metallic inclusions, sulfide distribution or morphology, for example, the sample is best examined in the as-polished condition since etching can remove various inclusions and attack structures such as ferrite stringers in an austenitic matrix.
On the other hand, if the search is designed to examine grain size, precipitation or cold- work deformation, the sample must be etched.
The condition of an alloy and its heat treatment play an important part in the selection and application of etchants. After confirming the alloy grade and analysis, consider whether it has been annealed, aged, cold worked, tempered and/or is in the as-hardened condition.
Highly Corrosion Resistant Alloys
Special techniques are required to effectively prepare highly corrosion resistant alloys for microstructural examination. Prominent in this group are alloys such as Micro-Melt® BioDur® CCM Plus®, MP35N* (UNS R30035) and Custom Age 625 Plus® (UNS N07716).
*MP35N is a registered trademark of SPS Technologies Inc.
These nickel-base and cobalt-base alloys, with their superior corrosion resistance, are etched using Waterless Kalling’s, Glyceregia, Acetic Glyceregia and Ralph’s. If the etchants typically used for highly resistant stainless and high temperature alloys do not work, HCL + H2O2 can be used. These alloys usually should be etched slowly.
All require a fresh polish to avoid “flashing,” which would require a complete re-polish. Best results are obtained by lightly re-rubbing one or two samples at a time on the final polishing wheel (normally a 0.05 micron alumina slurry).
The challenge with these alloys is to offset their natural tendency to self-passivate rapidly in the presence of oxygen. To effectively process these grades, etching must be done immediately after final polishing. Rinsing, drying and etching should be performed without delay.
When etching these highly resistant alloys, the etchant should be prepared in advance of the polishing process. This advance planning will minimize the length of time between polishing and etching, thus permitting a more effective microstructural evaluation. Samples should be polished and etched individually rather than processed in batches.
Microscopy Methods
Five light microscopy methods of illumination can be used in microstructural examination: bright-field illumination, dark-field illumination, oblique illumination, differential interference-contrast (DIC) and polarized light (Fig. 1). The well-equipped laboratory should have all five because their capabilities are complementary. Their role in microstructural evaluation cannot be overestimated.
Visual results for the same optical field at 100x magnification are shown via different lighting techniques.
 |
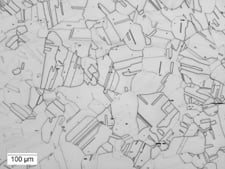 |
| (a) Stain etchant used to produce color | (b) Bright field – typical way of examining microstructure, shows grain structure and “twins,” but some areas not clearly defined |
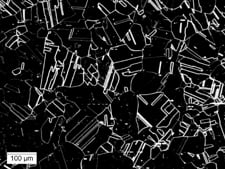 |
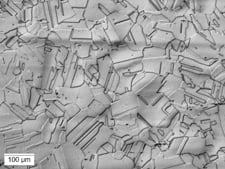 |
| ((c) Dark field – shows negative effect with grain boundaries more distinct, but some other areas not totally defined | (d) Three-dimensional effect makes grain boundaries clearer, with more sharply defined grains and “twins.” |
- Samples (b), (c) and (d) were etched using Waterless Kalling’s Reagent.
- Fig. 1. Light optical microscopy methods of illumination used in microstructural examination.
|
Light Optical Microscopy Methods |
|
|
Bright-Field Illumination |
The most commonly encountered method of illumination in which the light reflection is perpendicular to the specimen being viewed. Generally, microstructural features such as grain boundaries are dark and matrix regions are bright. |
|
Dark-Field Illumination |
The light is obliquely reflected back through the objective so that what appears bright and dark in bright-field illumination is reversed in dark-field illumination. |
|
Oblique Illumination |
The illustration source is de-centered at an oblique angle producing shadows on microstructural features. This method is extremely helpful if the operator knows the illumination direction; thereby, knowing which features are raised and which are recessed by the shadow orientation. |
|
Differential Interference-Contrast (DIC) |
A beam-splitting prism, polarizer and analyzer are inserted into the light path producing shadowing variations that reveal height differences in the microstructural features. |
|
Polarized Light |
The light is passed through a polarizing filter and can be adjusted to enhance the color contrast obtained with stain etchants. |
DIC, using bright-field microscopes that change the way light is deflected, gives a three-dimensional image. It is the ultimate in light optical microscopy, showing grain boundaries very clearly in highly corrosion resistant alloys. It is the best performing means of showing relief in structure, and is capable of identifying the effects of cold work, mechanical damage, etc. Compared with the other methods, DIC provides increased image contrast.
Dark-field, oblique and differential interference-contrast illumination methods can aid in delineating grain boundaries and other microstructural features that are only weakly visible in bright field illumination. A polarizing filter can be used in conjunction with a stain etch to enhance color contrast.
In dark-field technology, the grain seen is dark and the grain boundaries, light. With the bright-field microscope, the grain is light and the boundaries, dark. Oblique illumination brings light in on an angle, producing shadows on microstructural features.
Stainless Steels
When etching austenitic stainless steels, DIC illumination will better show the grain structure and help find cold-work deformation, when such exists. Waterless Kalling’s reagent can be used to show the general structure of many austenitic stainless alloys. Other agents such as Glyceregia or Acetic Glyceregia may be required to retain ferrite, carbide precipitation.
If the only interest is grain structure in an austenitic stainless, start etching with a more aggressive etchant. However, start etching with Glyceregia for a shorter time if interested in features other than grain boundaries, such as carbides, ferrite stringers, second phases and duplex structure.
Ralph’s reagent normally provides a good etch for general structures in ferritic stainless steels. Waterless Kalling’s and Glyceregia also can yield good results. Even after a good polish, scratches may still be visible. They may or may not be a problem.
Ralph’s is usually best for the precipitation hardenable stainless steels; however, Vilella’s reagent will work fine if a light etch is preferred. Etching time will vary because the alloy in the aged condition will react quicker to the etchant. The higher the aging temperature, the quicker the response. An alloy aged at 1100°F (590°C) will etch darker and quicker than one aged at 900°F (480°C).
The annealed structure in PH stainless steels requires either an aggressive etchant for a short time or a less aggressive etchant for a longer time. Here, Ralph’s reagent could be used for a few seconds, or Vilella’s, which is less aggressive, for a longer time.
Vilella’s reagent is preferred for martensitic stainless steels. Etching time and response will vary depending on whether the alloy has been annealed, hardened or tempered. Annealed samples usually require the longest etching time because everything is in solution, with not much to be seen. It is best to stop etching while the specimen is still on the light side. Etching has gone too long when the sample starts going black. A little experience will help the examiner to stop etching at the right time. Hardened and tempered samples usually require less etching time than annealed stock.
High Temperature Alloys
Etching procedures for high temperature alloys can vary greatly depending on condition of the material and what evaluation is required. High temperature alloys, which exhibit different aging conditions with a range of aging responses, phases, precipitates, etc., are typically more difficult to etch than austenitic stainless steels.
If uncertain about which etchant to use, start with Glyceregia and increase in severity until the desired result is obtained. This procedure requires only the final polishing step between etchants, instead of a complete re-polish. Glyceregia is mixed at time of use, and becomes more aggressive with the passage of time.
Waterless Kalling’s, another choice, works well with alloys such as Waspaloy (UNS N07001), 718 (UNS N07718) and A-286 (UNS K66286). It can be stored, and is thus more convenient to use.
Stain etchants and electrolytic etchants can be used to show specific aspects of structure in high temperature alloys. Stain etchants, used with immersion techniques, have been effective in highlighting structural features such as second phases.
If the etchants typically used for high temperature alloys do not work, HCL + H2O2 can be used just as they can for stainless steels. Precipitation can be evaluated using bright field microscopy, but DIC is better for showing grain structure.
Other Alloy Families
Nital (2 to 5 percent) is useful for showing carbide structure in tool and alloy steels, but re-etching the treated sample afterward with Vilella’s darkens the matrix and provides a clearer view of the microstructure. Many times, samples can be etched using Nital, examined and then etched with Vilella’s without re-polishing. Alloy condition will determine etching time.
Most magnetic and expansion alloys can be treated as suggested for one of the stainless steel families, or the tool and alloy steels. When examining the Ni-Fe alloys, for example, use the procedures for austenitic stainless steels. Many of the alloys used for their D.C. magnetic properties (such as stainless Type 430F) can be etched using procedures for ferritic stainless steels. Others, such as Fe and Si Core Irons, can be etched like low alloy steels using Nital.
Valuable Tips
Some etchants, like Waterless Kalling’s, can be made in bulk and stored for use as needed. This is convenient for the examiner. Other reagents, like Glyceregia and Acetic Glyceregia, must be prepared each time they are required. Both Glyceregia solutions undergo a continuous chemical reaction as they age, becoming aggressive in less than one hour. Special care must be taken then because the aggressive solution can affect etching time and procedure. It is a good idea, therefore, for the examiner to use the solution as soon as possible after mixing it.
As shown in Tables 1 and 2, many different etchants can be used successfully for specific alloys and special circumstances when examining basic microstructure. The choice of which reagent to use, however, does not have to be difficult.
Table 1 List of Etchants
|
Etchant No. |
Etchant Name |
Composition |
Remarks |
ASTM E 407 Designation |
|
1 |
Nital (2%) |
2cc HNO3 + 98cc Ethyl alcohol |
Immersion |
74 Nital |
|
3 |
Picral (5%) |
5gr Picric acid + 100cc Ethyl alcohol |
Immersion |
76 Picral |
|
4 |
Oxalic acid |
10gr oxalic acid + 10cc H2O |
Electrolytic at 200/400 Ma. |
|
|
6 |
Nital (5%) |
5cc HNO3 + 95cc Ethyl alcohol |
Immersion - Do Not Store |
74 Nital |
|
7 |
HCl in alcohol |
15cc HCl + 100cc Ethyl alcohol |
Immersion |
|
|
8 |
Ferric Chloride |
5g Ferric Chloride + 50cc HCl +100cc H2O |
Use Fresh Swab - Use Under Hood - Do Not Store |
|
|
9 |
Marble's Reagent |
4g CuSO4 + 20cc HCl + 20cc H2O |
Immersion or Swab |
25 Marble's |
|
10 |
Viella's |
5cc HCl + 2gr Picric acid + 100cc Ethyl alcohol |
Immersion or Swab |
80 Vilella's (ASTM contains 1gr Picric) |
|
11 |
Aqua Regia in alcohol |
100cc HCl + 3cc HNO3 + 100cc Methyl alcohol |
Immersion |
12 Aqua Regia |
|
12 |
Chromic acid |
10gr CRO3 + H2O |
Electrolytic at 200/400 Ma. |
|
|
13 |
2% H2SO4 |
2cc H2SO4 + 98cc H2O |
Use Electrolytic - Under Hood - 200/400 Ma. |
|
|
15 |
G |
12cc H3PO4 + 41cc HNO3 + 47cc H2SO4 |
Use Electrolytic - Under Hood - 200/400 Ma. |
|
|
18 |
Acetic Glyceregia (Mixed Acids) |
15cc HCl + 10cc HNO3 + 10cc Acetic Acid + 2/3 Drops Glycerine |
Use Fresh - Under Hood - Swab - Do Not Store |
|
|
19 |
Waterless Kalling's |
5gr CuCl2 + 100cc HCl + 100cc Ethyl alcohol |
Immersion or Swab |
95 Kalling's 2 |
|
22 |
HF + HNO3 |
1 to 3cc HF + 2 to 6cc HNO3 + 100cc H20 |
Swab - Handle with care - HF cause serious burns - Use in plastic container HF attacks glass |
|
|
23 |
HNO3 + H2O |
75cc HNO3 + 25cc H20 |
Use Under Hood - Electrolytic 5 to 7 amps |
|
|
26 |
Glyceregia |
15cc HCl +10cc Glycerol + 5cc HNO3 |
Use Fresh - Under Hood - Swab - Do Not Store |
87 Glyceregia |
|
28 |
Ralph's |
100cc H2O + 200cc methyl alcohol + 100cc HCl + 2gr CuCl2 + 7gr FeCl2 + 5cc HNO3 |
Swab |
|
|
29 |
Special #4 |
10% Sodium meta-Bisulfate in distilled water |
Immersion |
|
|
30 |
Special #5 |
20ml HCl + 4ml H2O2 (3%) |
Swab |
|
Note: Please see ASTM for proper handling of all chemicals.
Table 2 List of Alloys
|
Type |
Etchant |
Applications |
Special Notes |
|
10, 28, 19 |
General Structure - grain size, carbides, martensite and ferrite |
|
|
|
CarTech 10, CarTech 26 |
General structure - grain boundaries, carbides and martensite |
|
|
|
|
10, 19 |
General Structure - grain size, carbides, martensite and ferrite |
|
|
28 |
Excellent sulfide retention |
|
|
|
19, 26, 4, 12, 28 |
General Structure - grain boundaries, grain size, carbide precipitation |
|
|
|
|
10, 26, 4 |
General Structure - grain boundaries, grain size, carbide precipitation |
|
|
|
28 |
General Structure - grain boundaries, grain size. Excellent sulfide retention. |
|
|
26, 10 |
Similar to #11 but sulfides attacked |
|
|
|
10, 19, 18 |
General Structure |
|
|
|
|
6, 26, 10 |
General Structure - grain boundaries and grain size |
|
|
19, 26 |
General Structure |
|
|
|
Custom 450®, Custom 455®, Custom 465®, Custom 475®, Custom 630, 15Cr-5Ni, 13Cr-8Ni |
28 |
General Structure - martensite and austenite |
|
|
28, 19, 26 |
General Structure - grain boundaries, grain size, carbide precipitation |
|
|
|
V-57
|
19, 26, 9 |
General Structure, including size and grain boundaries |
|
|
13 |
Gamma prime precipitates - banding and depletion |
|
|
|
NCF 3015(1), Nickel 200/201, Thermo-Span, 720, 925 |
19, 26 |
General structure including grain size and grain boundary precipitate. |
|
|
19. 26 |
General structure including grain size and grain boundary precipitate. |
|
|
|
13 |
Gamma prime precipitates - banding and depletion |
|
|
|
860 |
19, 26, 15 |
General Structure, including size and grain boundaries |
|
|
13 |
Gamma prime precipitates - banding and depletion |
|
|
|
19, 26, 15 |
General Structure, including size and grain boundaries |
Generally 19 is more effective for aged material and 15 for solution treated material for general structure and grain size. |
|
|
13 |
Gamma prime precipitates - banding and depletion |
|
|
|
19, 26, 18 |
General Structure |
Use 18 in the annealed condition |
|
|
10 |
General Structure |
|
|
|
Ti Base Alloys |
22, 23 |
General Structure |
|
|
MP35N(2), Alloy 2 (AMS 5842)(3), MP35N LTi |
30 |
General Structure |
|
|
30 |
General Structure |
Fresh sample needed. Should prepare one sample at a time. Use of Differential Interference Contrast (DIC) would help. |
|
|
26, 18, 19 |
General Structure |
Fresh sample needed. Should prepare one sample at a time. Use of Differential Interference Contrast (DIC) would help. |
|
|
26 |
General Structure |
|
|
|
28 |
General Structure |
|
|
|
28 |
General Structure |
|
|
|
19, 29 |
General Structure |
|
|
|
19, 29 |
General Structure |
|
|
|
18Ni - 200 Maraging Steel, 18Ni - 250 Maraging Steel, 18Ni - 300 Maraging Steel |
12 |
Prior austenitic grain boundaries |
|
|
Cr-Fe, Glass Sealing 18, Glass Sealing 27, Glass Sealing 430F, Glass Sealing 446 |
11, 26, 28, 7, 8 |
General Structure, grain size |
|
|
28, 26, 19 |
General Structure |
|
|
|
Core Irons |
6, 11, 7 |
General Structure |
|
|
Fe and Si Core Irons |
11, 7 |
General Structure |
|
|
Ni-Cr-Fe - 22-3, 45-5, 42-6 |
28, 26, 19 |
General Structure |
|
|
Co-Fe Hy-Sat Alloy 27, Co-Fe-V Remendur, Hiperco® 50 |
26, 28, 19 |
General Structure |
|
|
Fe-Cr-Al, No. 1 No. 1 JR® Types 1 and 2 |
7, 11, 28, 26, 19 |
General Structure |
|
|
W1, O2, L6, D3, A2, A6, D2, T1, M2, M4, M42, H11, H12, H13, H21, M50 |
1, 6, 3, 11 |
General Structure |
|
|
10 |
Prior austenitic grain boundaries |
|
|
|
52100 |
1 |
General Structure |
|
(1) Manufactured and sold under license from Hitachi.
(2) Registered trademark of SPS Technologies, Inc.
(3) Also known as MP159N, a trademark of SPS Technologies Inc.
(4) Manufactured and sold under license from QuesTek Innovations LLC. Ferrium® is a registered trademark of QuesTek Innovations LLC.
With a little experience and a good understanding of what features need to be evaluated, the examiner may find that a few etchants will serve the intended purpose the majority of the time. In order of increasing strength, those etchants are: Nital, Villelas, Ralph’s, Glyceregia, Acetic Glyceregia, Waterless Kalling’s, and Hydrochloric Acid + Hydrogen Peroxide.
Health and Safety
All the referenced etchants are designed to attack the surface of the steels and alloys being analyzed. Therefore, all the etchants contain chemicals that are hazardous to handle, store, use and dispose of afterwards.
The user of these etchants is responsible for developing appropriate procedures to address all hazardous aspects of use as required by appropriate health, safety and environmental practices and regulations. Users should consult individual chemical Material Safety Data Sheets (MSDS’s) as a starting source for information. Users are further cautioned that mixtures of the individual chemicals may pose different and possibly more significant risks which they are obliged to assess prior to use. Standard etchant texts contain additional safety and use information.
***
By Katharine B. Small, David A. Englehart and Todd A. Christman
Carpenter Technology Corp., Wyomissing, PA, USA