Properties of an Essentially Nickel-Free Stainless Alloy for Medical Implants
White Papers
 |
| Medical screws manufactured from Carpenter's Carpenter Technology BioDur 108 alloy |
For some time, the medical device industry has expressed interest in a biocompatible, nickel-free stainless steel as an alternative to the alloys commonly used in implantable orthopedic devices such as bone plates; bone screws; fracture, trauma and spinal fixation components; hip and knee parts and other medical instruments currently manufactured from conventional nickel-containing alloys. In addition to biocompatability, these applications typically require high levels of strength, toughness, corrosion and fatigue resistance.
This article will describe pertinent properties of a new, essentially nickel-free stainless steel known as Carpenter Technology BioDur® 108 alloy (ASTM F2229-02) which has been developed by Carpenter Technology in response to this industry interest. It can be considered as an alternative to the three austenitic stainless alloys most commonly employed for these critical parts and components – Carpenter Technology BioDur Type 316LS alloy (ASTM F138), Carpenter Technology BioDur 22Cr-13Ni-5Mn alloy (ASTM F1314) and Carpenter Technology BioDur 734 alloy (ASTM F1586) (Fig. 1).
Figure 1. Comparative Chemistries of Four Carpenter Technology BioDur® Alloys
|
Element |
||||
|
C |
0.08 max. |
0.03 max. |
0.03 max. |
0.08 max. |
|
Mn |
21.00-24.00 |
2.00 max. |
4.00-6.00 |
2.00-4.75 |
|
Si |
0.75 max. |
0.75 max. |
1.00 max. |
0.75 max. |
|
P |
0.03 max. |
0.025 max. |
0.040 max. |
0.025 max. |
|
S |
0.01 max. |
0.010 max. |
0.030 max. |
0.01 max. |
|
Cr |
19.00-23.00 |
17.00-19.00 |
20.50-23.50 |
19.50-22.00 |
|
Ni |
0.05 max. |
13.00-15.50 |
11.50-13.50 |
9.00-11.00 |
|
Mo |
0.50-1.50 |
2.25-3.50 |
1.50-3.00 |
2.00-3.00 |
|
Cu |
0.25 max. |
0.50 max. |
-- |
0.25 max. |
|
N |
>0.90 |
0.10 max. |
0.20-0.40 |
0.25-0.50 |
|
Nb |
-- |
-- |
0.10-0.30 |
0.25-0.80 |
|
V |
-- |
-- |
0.10-0.30 |
-- |
Nominal chemical analyses of four alloys compared (Values = wt. %, Bal. = Fe)
In tests meeting ASTM standards, Carpenter Technology BioDur 108 alloy has exhibited significantly higher strength, in both annealed and cold worked conditions, than any of the common nickel-containing stainless alloys used in medical applications, including the three alloys compared in this study. It has demonstrated corrosion resistance essentially equivalent to that of Carpenter Technology BioDur 734 alloy and Carpenter Technology BioDur 22Cr-13Ni-5Mn alloy, and significantly greater than that of the widely used Carpenter Technology BioDur 316LS alloy. Finally, biocompatibility test results for Carpenter Technology BioDur 108 alloy proved favorable in every respect, qualifying the alloy as a candidate material for biomaterial applications.
Test Methods
Conventionally cast electro-slag remelted (ESR) ingots of Carpenter Technology BioDur 108 alloy were converted into billet for subsequent processing into finished bar and wire of various sizes. Tests were then conducted in the annealed and various cold worked conditions in comparison with the three studied alloys in equivalent metallurgical conditions.
Specimens were machined and tested in accordance with ASTM standards to measure mechanical properties including yield strength, tensile strength, impact toughness and fatigue resistance. Corrosion resistance was evaluated by calculating and measuring critical crevice corrosion temperature (per ASTM G48, Method D) and by completing PREN and pitting tests per ASTM G48 Method A.
Biocompatibility evaluations of test samples were conducted in accordance with ISO and industry standards. They included cytotoxicity, irritation, acute systemic toxicity, pyrogenicity, mutagenicity, implantation with histopathology and hemocompatibility tests.
Strength Properties
Carpenter Technology BioDur 108 alloy depends primarily on its high nitrogen content of approximately 1 percent to maintain its austenitic structure1. The high nitrogen also contributes to the alloy’s high levels of strength, ductility and corrosion resistance.
[1 Heat treating this alloy in non-argon atmospheres results in the formation of a thin magnetic (ferritic) surface layer on the heat treated product. This surface layer must be removed from the finished product prior to its use as a medical or surgical device.]
The typical yield strength of Carpenter Technology BioDur 108 alloy is approximately 606 MPa (88 ksi) in the annealed condition. In comparison, typical yield strength for Carpenter Technology BioDur 316LS is approximately 241 MPa (35 ksi) which is typical of austenitic stainless steels with low nitrogen content. The nitrogen-strengthened Carpenter Technology BioDur 734 and 22Cr-13Ni-5Mn alloys, with more nitrogen than Carpenter Technology BioDur Type 316LS alloy but less than Carpenter Technology BioDur 108 alloy, typically exhibit approximately 448 MPa (65 ksi) yield strength in the annealed condition.
The high nitrogen content of Carpenter Technology BioDur 108 alloy enhances the effect of cold work to increase strength levels. This effect can be seen in the tables showing yield strength and tensile strength achieved with varying amounts of cold work (Figs. 2 & 3).
Figure 2. Comparative Yield Strength (ksi)
|
Alloy |
% Cold Work |
|||||
|
Anl |
35% |
50% |
60% |
70% |
80% |
|
|
35 |
115 |
120 |
128 |
130 |
137 |
|
|
65 |
170 |
190 |
215 |
230 |
-- |
|
|
88 |
197 |
243 |
260 |
268 |
270 |
|
Yield strength of four studied alloys with increasing level of cold work.
*Carpenter Technology BioDur 734 essentially equivalent
Figure 3. Comparative Tensile Strength (ksi)
|
Alloy |
% Cold Work |
|||||
|
Anl |
35% |
50% |
60% |
70% |
80% |
|
|
120 |
190 |
215 |
230 |
245 |
-- |
|
|
135 |
230 |
270 |
292 |
308 |
320 |
|
Tensile strength of compared alloys with increasing level of cold work.
*Carpenter Technology BioDur 734 essentially equivalent
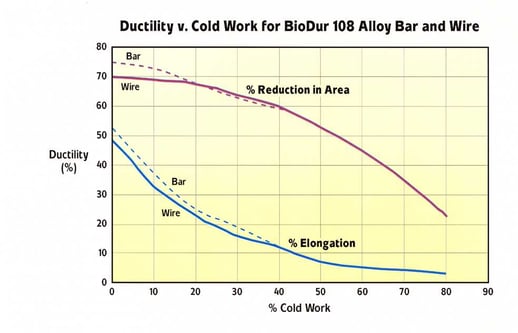
A graphical representation of the effect of cold reduction on yield strength for Carpenter Technology BioDur 108 compared with Carpenter Technology BioDur 734, Carpenter Technology 22Cr-13Ni-5Mn and Carpenter Technology BioDur 316LS is presented in Figure 4. As can be seen, Carpenter Technology BioDur 108 exhibits a yield strength approximately 20% higher than Carpenter Technology 22Cr-13Ni-5Mn and over double that of Carpenter Technology BioDur 316LS at a commonly used level of cold reduction (69%). Figures 5a and 5b summarize the four commonly reported mechanical properties of yield strength, tensile strength, elongation and reduction of area versus percent cold work reduction.
Figure 4. Comparative Yield Strength for Four Carpenter Technology BioDur® Alloys
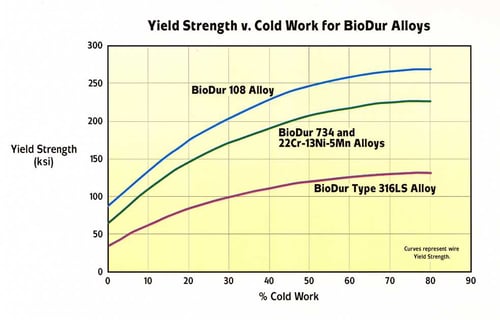
Figure 5a. Carpenter Technology BioDur® 108 Yield and Tensile Strength with Increasing Cold Work
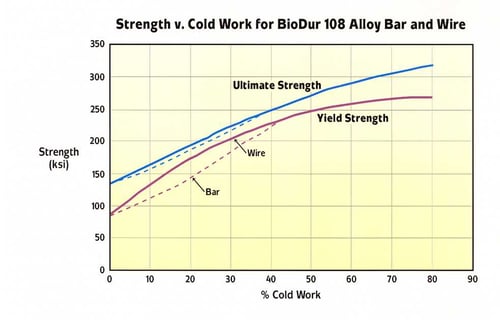
Figure 5b. Carpenter Technology BioDur® 108 Elongation/Reduction in Area with Increasing Cold Work
Like most austenitic alloys, Carpenter Technology BioDur 108 alloy has very high toughness. In fact, in the annealed condition, room-temperature impact energy levels for standard 10 mm x 10 mm Charpy V-notch specimens exceed the capacity of common testing machines. For this reason sub-size 10mm x 5mm Charpy V-notch impact specimens are suggested when testing for impact energy.
High-nitrogen austenitic alloys like Carpenter Technology BioDur 108 tend to exhibit "ductile-to-brittle" transition behavior, which is similar to that of ferritic alloys. In Carpenter Technology BioDur 108 alloy the ductile-to-brittle transition response is suppressed to below 0ºC. This transition takes place at approximately –20ºC. Therefore, Carpenter Technology BioDur 108 alloy can be considered for applications above –20ºC.
Carpenter Technology BioDur Type 316LS alloy was not tested for impact resistance in this study since the impact energy behavior of this commonly used alloy is well known.
While increased nitrogen in Carpenter Technology BioDur 108 alloy helps the material to achieve very high levels of strength, high nitrogen also enables this alloy to develop very high levels of fatigue resistance. Rotating-beam fatigue tests were conducted on specimens of the alloy from annealed bar stock having an ASTM #5 grain size and an ultimate tensile strength of 930 MPa (135 ksi). The fatigue limit observed was approximately 380 MPa (55 ksi), or about 41% of the ultimate strength. This fatigue limit is essentially equivalent to that found in Carpenter Technology 22Cr-13Ni-5Mn alloy and higher than that found in Carpenter Technology BioDur 316LS.
Corrosion Resistance
Corrosion resistance in austenitic alloys is strongly related to their chromium, molybdenum and nitrogen levels. Since these levels are high in Carpenter Technology BioDur 108 alloy, the material offers excellent resistance to pitting and crevice corrosion – which is critical for materials used in the manufacture of many medical devices.
Carpenter Technology BioDur 108 alloy was compared with 22Cr-13Ni-5Mn and Carpenter Technology BioDur 316LS alloys in terms of calculated Pitting Resistance Equivalent Number (PREN), measured weight loss in ferric chloride pitting tests (ASTM G48 Method A), and in calculated and measured critical crevice corrosion temperature (CCT) tests (ASTM G48 Method D). Carpenter Technology BioDur 108 alloy had a higher calculated PREN, the least amount of weight loss in pitting tests, and the highest calculated and measured CCT. See the table in Figure 6 for detailed results.
Figure 6. Comparative Corrosion Properties
|
Alloy |
PREN1 |
Pitting |
CCT3 (calc) |
CCT (meas) |
|
31 |
0.0 mg |
9ºC |
10ºC |
|
|
30.3 |
0.2 mg |
6.3ºC |
5ºC |
|
|
27.4 |
na |
-7.6ºC |
-5ºC |
*Carpenter Technology BioDur 734 essentially equivalent
1 - Pitting Resistance Equivalent (PREN) = Cr + 3.2Mo + 8N
2 - Test completed in 6% FeCl3per ASTM G48 Method A
3 - Critical Crevice Corrosion Temperature completed in 6% FeCl3+
1% HCL (ASTM G48 Method D)
*Carpenter Technology BioDur 734 essentially equivalent 1 - Pitting Resistance Equivalent (PREN) = Cr + 3.2Mo + 8N2 - Test completed in 6% FeClper ASTM G48 Method A3 - Critical Crevice Corrosion Temperature completed in 6% FeCl + 1% HCL (ASTM G48 Method D)
Testing for corrosion fatigue limits in distilled water solution and a standard Ringer’s solution at 37°C (98°F) have confirmed the relative corrosion resistance of the compared alloys as stated above. In addition, Carpenter Technology BioDur 108 alloy passed the ASTM A262 Practice A requirements for resistance to intergranular corrosion.
Biocompatibility
Carpenter Technology BioDur 108 alloy was also subjected to a series of biocompatibility tests to further evaluate the alloy for potential use in biomaterial applications, and to compare it with the nickel-bearing austenitic stainless steels most commonly used. Extensive biocompatibility tests were conducted by Toxikon Corp., Bedford, MA, on behalf of Carpenter. Carpenter Technology BioDur 108 alloy met all the requirements of the test standards used. Briefly defined here are highlights of the findings; more specific details about test procedures and standards met (such as ISO numbers) are available from Carpenter.
Cytotoxicity: The alloy was found to be non-cytotoxic and meeting the requirements of the Elution test, ISO 10993. The alloy was found to be non-cytotoxic and meeting the requirements of the Elution test, ISO 10993. Irritation: Test samples did not exhibit any signs of erythema, edema or necrosis. Alloy was concluded to be a negligible irritant. Test samples did not exhibit any signs of erythema, edema or necrosis. Alloy was concluded to be a negligible irritant.
Acute Systemic Toxicity: No signs of toxicity were observed. Test samples met the requirements of ISO 10993-11, Systemic Injection Test. No signs of toxicity were observed. Test samples met the requirements of ISO 10993-11, Systemic Injection Test. Pyrogenicity: Test samples met the requirements of ISO 10993-11 for the absence of pyrogens. Test samples met the requirements of ISO 10993-11 for the absence of pyrogens.
Mutagenicity: Alloy samples were concluded to be non-mutagenic based on the test methods employed. Alloy samples were concluded to be non-mutagenic based on the test methods employed. Implantation with Histopathology: No signs of toxicity were exhibited after 14- and 28-day implantation test periods. Alloy was concluded to be non-toxic.
No signs of toxicity were exhibited after 14- and 28-day implantation test periods. Alloy was concluded to be non-toxic. Hemocompatibility: Alloy samples were concluded to be non-hemolytic based on test methods employed. Alloy samples were concluded to be non-hemolytic based on test methods employed.
In summary, the essentially nickel-free Carpenter Technology BioDur 108 alloy has shown high levels of strength, impact toughness, fatigue and corrosion resistance in comparison with the more commonly used Carpenter Technology BioDur 22Cr-13Ni-5Mn/Carpenter Technology BioDur 734 and Carpenter Technology BioDur 316LS. For these reasons it can be considered as a possible material upgrade for biomedical implant and instrument applications
***
By Michael J. Walter, Medical Alloy Specialist
Carpenter Technology Corporation, Reading, PA